Trésor-Economics No. 199 - Strategies to expand the distribution of generic drugs
When a pharmaceutical company wants to market a new molecule, it applies for a patent. The patented drug, known as the "originator" drug, is generally protected for between 10 and 15 years. After the patent expires, the pharmaceutical company no longer has the monopoly on manufacturing the drug, and can face competition from generic pharmaceutical firms. In France, whether they are made by generic companies or the company producing the originator, generic drugs must be manufactured from the same active ingredient, at the same dosage level and with the same route of administration as the originator.
When a generic drug is introduced, it lowers prices in two ways: through the use of generic drugs that are less expensive than the originator (the regulated price for a generic is 60% lower than the initial price of the originator), and a drop in the price of the originator (an immediate regulated decrease of 20%).
Because they offer a medical service equivalent to that provided by the originator while decreasing the cost to the national health insurance fund, generic drugs are an ideal way to curb healthcare spending.
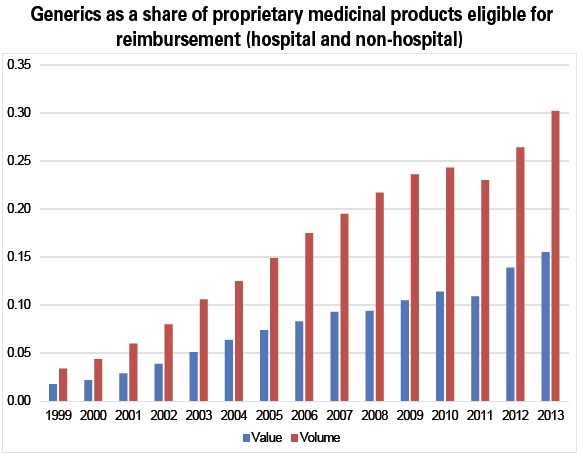
Certain measures encourage the substitution of generic drugs for originators (particularly the "third-party payer in exchange for generics" (tiers-payant contre génériques) system). Substitution has become increasingly popular in recent years, and by 2014 represented 73% of the volume of drugs for which generics can be substituted, and 66% of the value. According to the Government Audit Office, these efforts brought down spending on drugs by the national health insurance fund by some €1.6bn in 2013, net of the potential savings passed on to pharmacies (€1.8bn).
More could be done, however, to expand the use of generics. In France, generic drugs' share of total consumption of pharmaceuticals is nearly 1.6 times lower than the OECD average: the acceptable substitution rate of generics for originators (where such generics exist) is offset by the low number of prescriptions for drugs for which there are generic substitutes.
To increase the penetration of generics and thereby generate savings, the current system could be adapted in various ways:
- By lowering regulated prices even further, while ensuring that generic pharmaceutical companies remain viable. Various simulations result in savings of between €170m and €1bn.
- By new incentives for physicians to prescribe more generic drugs
Currently, two-thirds of the pharmaceutical products that are the most costly for the national health insurance fund are biologics rather than chemical drugs, and are not covered by the system that applies to generics. We therefore need to introduce a better governance framework for these drugs in order to generate the maximum possible savings when their patents expire.